Introduction: A Beacon of Hope from the FDA In the realm of medical breakthroughs, the FDA stands as a pillar of authority and reliability. Their recent endorsement of a groundbreaking RSV vaccine for pregnant individuals brings a glimmer of hope for expectant mothers and their precious newborns. Join us as we delve into the significance
Introduction: A Beacon of Hope from the FDA
In the realm of medical breakthroughs, the FDA stands as a pillar of authority and reliability. Their recent endorsement of a groundbreaking RSV vaccine for pregnant individuals brings a glimmer of hope for expectant mothers and their precious newborns. Join us as we delve into the significance of this vaccine, its potential to protect two lives, and the transformative impact it can have on the world of healthcare.
Meeting the Guardians of Health: The FDA’s Stance
When it comes to evaluating the safety and effectiveness of medical interventions, the FDA’s stamp of approval holds immense weight. With a track record of rigorous assessment and stringent standards, their endorsement of the new RSV vaccine speaks volumes. Backed by extensive research and clinical trials, this vaccine signifies a pivotal step forward in maternal and child health.
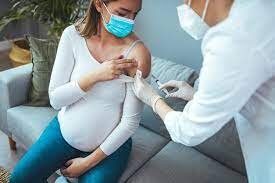
Image by: https://www.cidrap.umn.edu/respiratory-syncytial-virus-rsv/fda-advisers-recommend-rsv-vaccine-pregnant-women-protect-newborns
The RSV Threat: Beyond a Common Cold
Respiratory Syncytial Virus (RSV) is not just another cold; it’s a potent adversary that particularly threatens vulnerable populations like newborns and pregnant individuals. RSV infections can lead to severe respiratory distress, hospitalization, and in extreme cases, even be life-threatening. By focusing on protecting pregnant individuals, this vaccine serves as a shield for both the expectant mother and the unborn child.
Unveiling the Vaccine’s Mechanism
At the heart of this medical breakthrough lies a meticulously crafted vaccine. Designed to bolster the immune response of pregnant individuals, the vaccine prompts the body to produce antibodies against RSV. These protective antibodies are then passed on to the developing fetus, offering them a defense against RSV during their early months of life when they are most vulnerable.
Advantages Over Conventional Approaches
| Aspect | RSV Vaccine for Pregnant Individuals | Conventional Approaches |
|---|---|---|
| Dual Protection | Shields both the mother and the newborn. | Protects the mother, but not the newborn. |
| Early Immunity | Provides passive immunity to the fetus. | Immunity development post-birth. |
| Reduced Severity | Decreases the severity of potential infections. | Limited impact on infection severity. |
| Holistic Defense | Offers protection during the most vulnerable period. | Limited protection in early infancy. |
Navigating Challenges: From Research to Rollout
While the promise of the RSV vaccine is undeniable, its journey from research to widespread implementation isn’t without challenges. Ensuring the vaccine’s safety during pregnancy, addressing potential side effects, and establishing accessibility are crucial milestones that require collective effort from healthcare professionals, researchers, and policymakers alike.
Embracing a Healthier Future
For individuals seeking comprehensive healthcare solutions, the FDA’s endorsement of the RSV vaccine signals a shift toward a brighter, healthier future. By taking proactive measures during pregnancy, expectant mothers empower themselves and their newborns with an added layer of protection. This transformative approach aligns with the evolving landscape of healthcare – one that emphasizes prevention and well-rounded care.
Conclusion: Empowering Through Innovation
As we stand at the crossroads of medical advancement, the RSV vaccine for pregnant individuals emerges as a beacon of empowerment. The collaborative efforts of researchers, healthcare providers, and regulatory agencies have paved the way for a new chapter in maternal and child health. With each vaccine administered, we move closer to a world where the most vulnerable among us are safeguarded, cherished, and nurtured.














Leave a Comment
Your email address will not be published. Required fields are marked with *