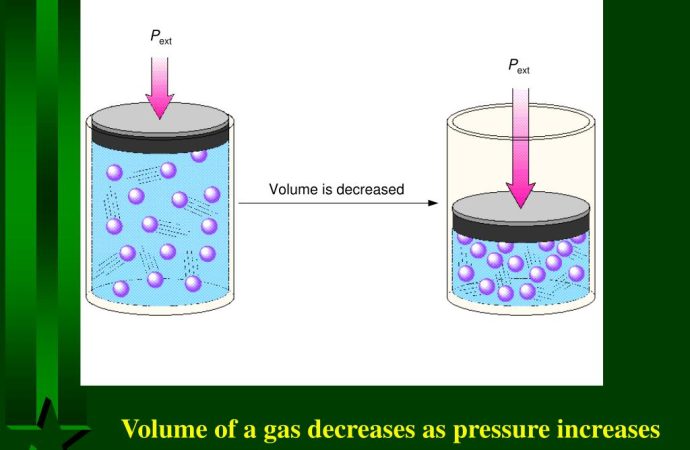
Thermodynamics is a fundamental branch of physics that deals with the study of energy and its transformations. It plays a crucial role in understanding various natural phenomena, including energy transfer and entropy. In this article, we will delve deeper into the concepts of energy transfer and entropy, exploring their significance and real-world applications. Introduction to
Thermodynamics is a fundamental branch of physics that deals with the study of energy and its transformations. It plays a crucial role in understanding various natural phenomena, including energy transfer and entropy. In this article, we will delve deeper into the concepts of energy transfer and entropy, exploring their significance and real-world applications.
Introduction to Thermodynamics
What is Thermodynamics?
Thermodynamics is the branch of physics concerned with the relationships between heat, work, and energy. It provides a framework for understanding how energy moves and changes form within systems.
Importance of Understanding Energy Transfer and Entropy
Energy transfer and entropy are essential concepts in thermodynamics that have wide-ranging implications in fields such as physics, chemistry, engineering, and biology. A comprehensive understanding of these concepts is crucial for various applications, from designing efficient energy systems to explaining natural processes.
Basic Concepts of Energy Transfer
Definition of Energy Transfer
Energy transfer refers to the process of energy moving from one system to another. It can occur in various forms, including heat transfer (thermal energy) and work transfer (mechanical energy).
Types of Energy Transfer
Heat Transfer
Heat transfer is the exchange of thermal energy between systems due to a temperature difference. It can occur through conduction, convection, or radiation.
Work Transfer
Work transfer involves the transfer of mechanical energy between systems through the application of force. It can result in the displacement or deformation of objects.
Laws of Thermodynamics
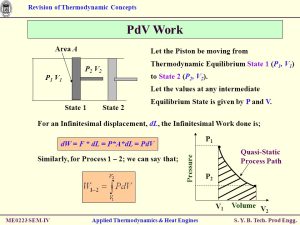
Image by : Yandex
The laws of thermodynamics govern the behavior of energy in systems. They include principles such as the conservation of energy, the increase of entropy, and the impossibility of reaching absolute zero temperature.
Understanding Entropy
Definition of Entropy
Entropy is a measure of the disorder or randomness of a system. It quantifies the number of possible arrangements of a system’s particles and is related to the system’s energy distribution.
Relationship Between Entropy and Disorder
Entropy tends to increase in isolated systems over time, leading to an increase in disorder or randomness. This phenomenon is described by the second law of thermodynamics.
Second Law of Thermodynamics and Entropy
The second law states that the entropy of a closed system always increases over time. It implies that natural processes tend to move towards states of higher entropy, leading to the degradation of energy and the eventual dissipation of useful energy.
Applications of Thermodynamics in Daily Life
Thermodynamic principles have numerous applications in everyday life, influencing the design and operation of various systems and processes.
Heating and Cooling Systems
Thermodynamics governs the operation of heating, ventilation, and air conditioning (HVAC) systems, which regulate indoor temperature and humidity levels.
Engines and Refrigerators
Thermodynamic principles are central to the functioning of engines, refrigerators, and heat pumps, where energy transfer and entropy play key roles.
Chemical Reactions
Chemical reactions, such as combustion and photosynthesis, are governed by thermodynamic principles, influencing reaction rates and product yields.
Importance of Energy Efficiency
Role of Thermodynamics in Improving Energy Efficiency
Thermodynamics provides insights into maximizing energy efficiency and minimizing energy waste in various processes and technologies.
Sustainable Energy Practices
Understanding thermodynamics is essential for developing sustainable energy practices that reduce environmental impact and promote resource conservation.
Real-world Examples of Energy Transfer and Entropy
Photosynthesis
Photosynthesis, the process by which plants convert sunlight into chemical energy, involves complex energy transfer mechanisms and entropy considerations.
Combustion
Combustion reactions, such as the burning of fossil fuels, release heat energy through chemical reactions, which are governed by thermodynamic principles.
Human Metabolism
Human metabolism relies on thermodynamic processes to convert food into energy for cellular activities, illustrating the relevance of thermodynamics to biological systems.
Challenges and Future Directions
Addressing Energy Transfer Inefficiencies
Efforts are underway to address energy transfer inefficiencies and develop more efficient technologies and processes through advances in thermodynamic research.
Advancements in Thermodynamic Research
Ongoing research in thermodynamics aims to deepen our understanding of energy transfer, entropy, and related phenomena, paving the way for future innovations in energy science and technology.
Conclusion
In conclusion, a solid grasp of thermodynamics is essential for comprehending energy transfer and entropy, which are fundamental concepts with broad applications in science, engineering, and everyday life. By enhancing our understanding of these principles, we can develop more efficient energy systems, mitigate environmental impacts, and advance sustainable practices for the benefit of present and future generations.
FAQs
1. Why is thermodynamics important?
Thermodynamics provides the foundation for understanding how energy moves and transforms within systems, influencing various natural and technological processes.
2. How does entropy relate to disorder?
Entropy is a measure of the disorder or randomness of a system. As entropy increases, the disorder within a system also increases.
3. What are some real-world examples of thermodynamic processes?
Examples include the operation of engines, refrigerators, chemical reactions, and biological processes such as metabolism and photosynthesis.
4. How does thermodynamics impact energy efficiency?
Thermodynamics informs strategies for maximizing energy efficiency and minimizing energy waste in various systems and processes, contributing to sustainability efforts.
5. What are the future directions of thermodynamic research?
Future research aims to address energy transfer inefficiencies, develop more efficient technologies, and deepen our understanding of thermodynamic phenomena for continued innovation in energy science and technology.