Introduction A historic change may be on the horizon for women’s healthcare in the United States. The Food and Drug Administration (F.D.A.) advisers are reviewing whether a birth control pill should be made available over the counter (OTC) without a prescription. If approved, this move could transform access to contraception, making it easier for millions
Introduction
A historic change may be on the horizon for women’s healthcare in the United States. The Food and Drug Administration (F.D.A.) advisers are reviewing whether a birth control pill should be made available over the counter (OTC) without a prescription. If approved, this move could transform access to contraception, making it easier for millions of women to take control of their reproductive health. Supporters believe it would break down barriers to care, while critics raise concerns about safety, proper usage, and the need for medical oversight.
Why This Decision Matters
For decades, birth control pills have required a prescription in the United States, often meaning women must visit a doctor or clinic for access. While this system ensures medical guidance, it can also create obstacles, especially for those without health insurance, those living in rural areas, or those with busy schedules.
If an OTC birth control pill becomes available, women could purchase it directly at pharmacies, much like buying pain relievers or vitamins. This increased accessibility could help reduce unplanned pregnancies and give women more flexibility in managing their health.
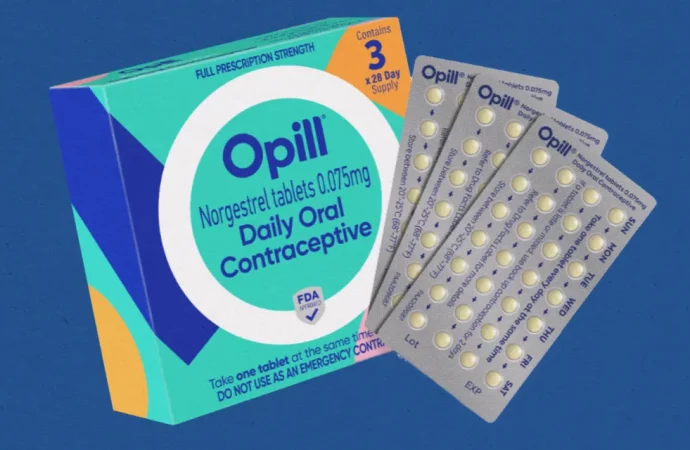
The Pill Under Consideration

Image by: Yandex.com
The pill being reviewed by F.D.A. advisers contains progestin, a hormone used in many birth control options. Progestin-only pills, sometimes called “mini-pills,” are considered safe for most women, including those who cannot take estrogen-based contraceptives.
These pills work by thickening cervical mucus and thinning the uterine lining, making it harder for sperm to reach an egg. They must be taken daily, ideally at the same time each day, to be most effective.
Support for Over-the-Counter Access
Many healthcare organizations, including reproductive rights groups, have long pushed for over-the-counter access to birth control pills. They argue that the science supports safety, and that medical oversight is not necessary for most users.
Studies show that women are capable of understanding the instructions and identifying whether they are good candidates for the pill. Making it OTC could also lower healthcare costs by reducing the need for doctor visits just to get a prescription.
Concerns and Cautions
Not everyone is fully supportive of the change. Some healthcare providers worry that without medical guidance, women might overlook potential side effects or health conditions that could make the pill less safe. For example, certain heart or liver issues might require alternative birth control methods.
There are also concerns about whether OTC availability could lead to reduced doctor visits, which might mean missing other important screenings, such as cervical cancer tests or blood pressure checks.
Lessons from Other Countries
Several countries already offer birth control pills without a prescription, including the United Kingdom, parts of Latin America, and New Zealand. In these countries, the move has generally been seen as successful, with high rates of safe usage and improved access.
Advocates point to these examples as proof that U.S. women could also benefit from easier access, especially with clear instructions and public health education.
Potential Impact on Women’s Lives
If the F.D.A. advisers recommend approval, and the pill becomes available OTC, it could have wide-reaching effects. Women who previously faced transportation challenges, cost barriers, or scheduling conflicts could gain more control over their reproductive health.
It could also be particularly beneficial for young women and teenagers who might be hesitant to visit a clinic for birth control. With proper education, this group could use the pill effectively while avoiding unintended pregnancies.
The Role of Education and Awareness
Making a birth control pill available without a prescription would need to be paired with strong educational efforts. Pharmacies and packaging would need to provide clear, simple instructions on how to take the pill, what side effects to watch for, and when to seek medical advice.
Public health campaigns could play a key role in ensuring that women understand the importance of consistency in taking the pill and the fact that it does not protect against sexually transmitted infections.
Balancing Access and Safety
The challenge for the F.D.A. and healthcare leaders is to find the right balance between making contraception more accessible and ensuring it is used safely. This decision will likely involve careful consideration of packaging, labeling, and public health outreach to support women in making informed choices.
The Path Ahead
The F.D.A. advisers’ recommendation is not the final step. After reviewing their input, the agency will make its official decision. If approved, the United States would join a growing list of countries offering OTC birth control pills—a move many see as a major milestone in women’s health rights.
This could also set a precedent for future healthcare decisions, showing that with the right safeguards, more medicines could be safely made available without prescriptions.
Conclusion
The possibility of an over-the-counter birth control pill marks a significant moment in the ongoing effort to expand women’s healthcare access. If approved, it could empower millions to take charge of their reproductive choices without unnecessary barriers. While safety and education remain critical, the potential benefits—especially for underserved communities—are immense. The F.D.A.’s final decision will shape the future of birth control accessibility in the United States and could redefine how women approach their health for years to come.














Leave a Comment
Your email address will not be published. Required fields are marked with *